Drug discoveries have always played a role in improving the quality of life and increasing the average lifespan of the human race.
With an increase in novel infections and chronic diseases, the need for new drugs is more crucial than ever now.
Experts believe that Artificial Intelligence (AI) can be the answer to creating new drugs that revolutionize the healthcare industry.
AI can make drug discovery, testing, and repurposing easier and more precise.
Keep reading to know AI's current status and future in drug discovery.
Did You Know?
Genes can affect how we respond to medications, including the drug's efficacy and the risk of side effects.
Introduction
Artificial Intelligence (AI) has started impacting various industries, including healthcare.
Drug discovery is an extensively time-consuming and expensive process that only very few pharmaceutical and biotechnology companies can afford to take up.
AI in drug discovery has the potential to affect the lives of millions around the world.
Experts now believe that AI can reduce the cost of drug discovery, speed up the process, and pave the way for transforming healthcare.
What Is Drug Discovery?
Drug discovery involves discovering new drugs or medications with the efforts of large pharmaceutical and biotechnology companies and governments.
With the results of drug discovery uncertain and the high costs involved in the process, traditional drug discovery comes with multiple challenges and bottlenecks.
Did you know the average cost of discovering a drug can be up to $2.6 billion?
From research to approvals and marketing, the average time needed to discover a drug is about 12 years.
Unfortunately, many patients don’t have this time.
The Stages Of Drug Discovery
A drug must go through these four stages to be released into the market.
- Early Discovery
The first stage of drug discovery is identifying a target and finding a possible lead that can affect or treat the target.
- Preclinical Trial
In this stage, the substances identified during the early discovery stage are tested in the lab for toxicity and efficacy. Animal testing and lab testing happen in this stage.
- Clinical Research
Four phases of clinical research happen after the preclinical trials are successful.
Phase I, II, and III are safety and efficacy tests performed on healthy human volunteers and patients.
The side effects, rate of improvement, and maximum tolerated doses are identified and recorded in these stages.
Phase IV is the post-marketing stage after approvals that monitors the side effects after the drug hits the market.
After the first three phases of clinical trials, the drug is submitted for approval.
- Review And Approval
A regulatory authority examines the results of the trials and drug-related documents and chooses to approve or reject the drug. The Food and Drug Administration (FDA) is one such regulatory body.
Challenges In These Methods
The following are some of the challenges in the traditional drug discovery process.
- Drug development is a very tedious and time-consuming process.
- Developing a single drug may cost billions of dollars.
- For certain diseases and health conditions, target identification gets very challenging.
- In the case of infectious diseases, the pathogens may change by the time drugs are discovered and approved.
- Animal testing, in many cases, does not help understand the full impact of the drug.
- Clinical approvals and regulatory processes are intensive. Out of 5,000 compounds that entered the preclinical stage, only five reached the stage of human testing, and only one received final approval.
How Can AI Be Used In Drug Discovery?
Artificial Intelligence (AI) is a term that refers to various computing technologies that can simulate human intelligence.
According to experts, AI can speed up drug discovery and help get the right drugs to needy people.
AI may also help handle the vast amount of data generated during every step of drug discovery.
Every stage of research and drug testing creates several terabytes of data.
Even though the researchers take years to analyze the data and find patterns and links, the sheer quantity of data available may lead to misses and overlooks.
Machine Learning (ML), a segment of AI, can help analyze large amounts of data precisely yet quickly and identify insights that may help better the drug.
Such insights can add value to the present and future drug discoveries.
With just one out of 5000 components reaching the approval stage, researchers spend a lot of time pursuing leads that may have severe or ineffective side effects.
AI can be trained to do a cost-benefit analysis and check historical data to predict the outcome of these leads.
This will save researchers a tremendous amount of time, resources, and effort.
Proposal submissions include extensive work, including collecting all the needed documents, putting them together, and having the correct answers to the authorities' questions.
AI can also be used to put together a tight case using predictions and historical analysis.
AI and the Promise of Personalized Medicine
For a long time now, medical professionals have considered the idea of personalized medicine and the benefits this may have on patients.
Personalized medicine is the idea of curating drugs based on the patient's physical, mental, genetic, and environmental features.
Personalizing drugs can improve the treatment's efficiency and reduce the risk of side effects.
Personalizing medicine for billions of people can only be possible with supportive technology like AI.
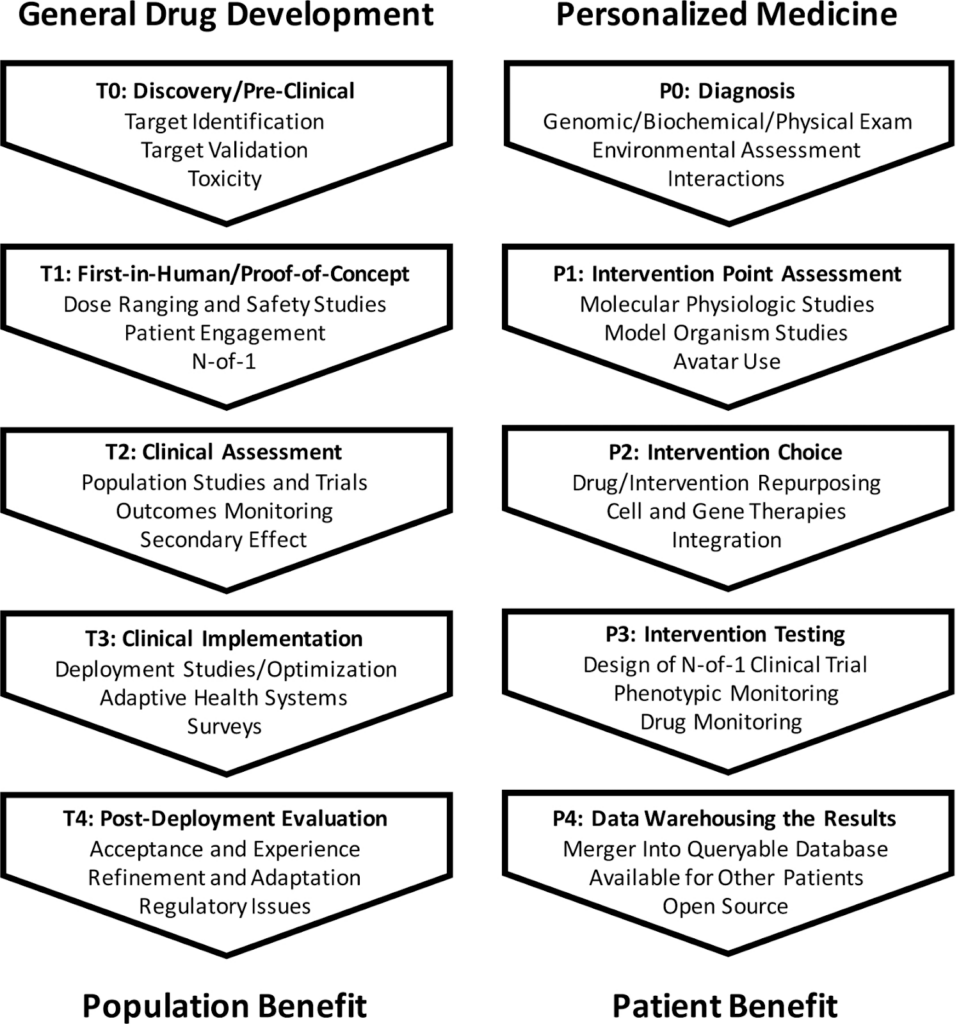
Image source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7580505/
(This image shows how different the steps of personalized medicine discovery would be compared to traditional drug discovery)
Success Stories Of AI In Drug Discovery
Many biotechnology and pharmaceutical companies have started experimenting with AI for drug discovery. Here are a few such success stories.
Atomwise
Atomwise is a pharmaceutical company based in San Francisco, California, using AI technologies to create small-molecule drugs.
The Atomnet model is a trademark drug discovery algorithm created by this brand based on deep convolutional neural networks.
This AI/ML-based algorithm does the following.
- Identify small molecule drugs for poor or unrecognizable targets
- Screen trillions of compounds quickly, helping eliminate unlikely leads
- Make accurate predictions and compare them with historical results
- Reproduce hundreds of historical experiments with precision
The company has used AI technology since 2013 for its drug discovery process.
BenevolentAI
BenevolentAI is an AI-based drug discovery and pharma company based in Luxembourg, Europe.
The Benevolent Platform is a trademarked AI platform of the brand that handles every step of drug discovery - from target identification to clinical development.
This AI platform has generated multiple drug possibilities, most in their lead optimization or preclinical stages.
BEN-2293, a possible drug for atopic dermatitis, has reached Phase III of clinical trials and will soon be submitted for approval.
The following are some of the features of the Benevolent Platform.
- Ability to work on any therapeutic area
- Can focus on both biological and antibody targets
- Ability to identify unrecognizable or poor targets
- Scalable
Overview Of The Current Regulations Surrounding AI In Drug Discovery
Regarding using AI for drug discovery, safety, and privacy are two factors everyone is worried about.
Significant deals and mergers have occurred between pharmaceutical, biotechnology, and tech companies in the last couple of years.
As a result, regulators worldwide are working on initiatives that would help combat some of the challenges of AI in drug discovery.
In 2022, the UK published a 10-year National AI Strategy that would regulate the use of artificial intelligence in different industries, including pharmaceuticals.
Canada proposed a national regulatory framework called the Artificial Intelligence and Data Act (AIDA) in 2022 to focus on trade and commerce using AI systems.
This would also encompass regulations regarding the use of AI for drug discovery.
The FDA has already issued an action plan named ‘Artificial Intelligence and Machine Learning in Software as a Medical Device’ in 2019 to monitor the use of AI by medical device manufacturers.
The FDA is right now focusing on regulating cybersecurity in the use of AI systems.
The Future Of AI In Drug Discovery
The advancement of AI combined with a better understanding of this technology will help finetune drug discovery in the future.
AI will make discovering ground-breaking drugs easier, quicker, and more effortful.
However, this would take time.
As more pharmaceutical and biotechnology companies invest in AI technologies, they will get closer to modernizing the drug discovery process.
Although no AI-developed drugs are available, companies are getting closer to achieving this.
Summary
- The traditional method of drug discovery is highly tedious, expensive, and time-consuming. Only one in 5,000 compounds end up getting sent for final approvals.
- Experts are now considering using Artificial Intelligence (AI) in drug discovery, hoping to reduce the time and cost of discovering new drugs and make the process more efficient.
- AI in drug discovery can help analyze the vast amount of data generated, eliminate ineffective leads, quicken the process, and predict the outcome of the discovery process.
- AI in drug discovery can also create personalized medicine, ensuring lower side effects and better treatment rates.
- Companies like Atomwise and BenevolentAI are already successfully working on AI-based drug discovery techniques and are standardizing the processes.
- Governments worldwide are creating regulations to handle the privacy and safety challenges of AI-based drug discovery.
- Pharma and biotech companies continue to invest in AI technologies. As a result, they are getting closer to modernizing drug discovery and creating safe and effective drugs using AI.
References
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7577280/
- https://www.nature.com/articles/d43747-021-00045-7
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5725284/
- https://pubmed.ncbi.nlm.nih.gov/26928437/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6117650/
- https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process
- https://www.ncbi.nlm.nih.gov/books/NBK195047/
- https://www.ncbi.nlm.nih.gov/books/NBK195047/table/table_2-1/?report=objectonly
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1299137/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7580505/
- https://www.gov.uk/government/publications/national-ai-strategy/national-ai-strategy-html-version#pillar-3-governing-ai-effectively\
- https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-software-medical-device
- https://www.nature.com/articles/d43747-021-00045-7
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7577280/
- https://hms.harvard.edu/news/can-ai-transform-way-we-discover-new-drugs