From a decade ago, when it cost a billion dollars to sequence the entire DNA content, to now getting it sequenced for a thousand dollars, genomic research has come a long way. And we’re not very far from having a smartphone app that’ll warn you of your health risks, suggest medications best suited for you, and even predict the health status of your unborn baby. This is all thanks to one thing: genomics! In this article, we explore how the field of medicine and healthcare has leveraged genomic information to offer effective and personalized solutions to a range of health conditions, from diabetes to cancer. We further touch upon the future of genomic medicine as a tool to revolutionize the healthcare system.
Did You Know?
Genomic medicine is not just employed in clinical settings but is now accessible to consumers so that they can learn in-depth about how their genes interact with health. For those who have already taken an ancestry genetic test, this information can easily be accessed in just 3 steps!
What is Genomic Medicine?
Our DNA is 3 billion letters long - 4 letters, A, T, G, and C, are repeated across the entire length. Sequencing is a technology that allows scientists to decipher the order in which the 4 letters are arranged.
Depending on the application, the entire 3 billion letters may be sequenced (whole genome sequencing), only the part of the DNA that makes protein may be sequenced (exome sequencing), or one particular gene may be sequenced (targetted sequencing).
Using the information from sequencing to improve clinical care and health outcomes through effective diagnosis and personalized treatment is known as genomic medicine.
Genomic medicine is already making huge impacts in many fields of medicine, including:
- Oncology
- Pharmacology
- Rare diseases
- Infectious diseases
All human beings are 99.9 percent identical in their genetic makeup.
Differences across individuals in the remaining ~0.1% hold significant clues about their health. Some differences may be harmless, while some others may contribute to disease risk.
With genomic medicine, it is possible to analyze these differences in clinical settings and compare them to many reference sequences.
This information can help understand whether the differences contribute to a disease and determine the best treatment option.
Basic Definitions in Genomic Medicine
| Term | Description |
| Biomarker | DNA or RNA nucleotides or bases are read in groups of three (e.g., ATG, AUG) called codons. Start and stop codons show when a protein sequence starts or ends. |
| Codon | DNA or RNA nucleotides or bases are read in groups of three (e.g., ATG, AUG), which are called codons. Start and stop codons show when a protein sequence starts or ends. |
| DNA | Deoxyribonucleic acid (DNA) is the carrier of genetic information. DNA consists of four nucleotides or bases (A, T, G, and C). DNA can replicate or make copies of itself. |
| Exome | The approximately 1% of the genome formed only by exons. |
| Exon | The protein-coding sequence of DNA (the part of the genome that is expressed). |
| Gene | A gene is a specified sequence of DNA that serves as the basic unit of heredity. “Gene” comes from the Greek word genea, meaning generation. |
| Gene expression | When a gene is turned on, and its RNA or protein product is being made, the gene is said to be expressed. The on/off state of cells is called a gene expression profile, with each cell type having a unique profile. |
| Genome | The genome includes all of an organism’s DNA, including both exons and introns. |
| Germline | Germline cells are sperm, egg, or embryo cells. Changes to the germline are permanent. Germline traits or mutations are inherited and generational. |
| Intron | The non-protein coding sequence of DNA (the part of the genome that is not expressed). |
| MicroRNA | MicroRNA (miRNA) is a type of genetic material that regulates gene expression. miRNAs are promising biomarkers and can point toward the development of new therapeutic approaches. |
| RNA | Ribonucleic acid (RNA) is a single-stranded copy of the DNA sequence that plays a messenger role in helping cells carry out instructions for making a protein. RNA consists of four nucleotides or bases (A, U, G, and C). The DNA T is replaced by the RNA U when copied. |
| SNP | A single-nucleotide polymorphism (SNP, pronounced “snip”) is a DNA sequence variation that occurs when a single nucleotide (A, T, C, or G) in a gene sequence is altered. SNPs are the most abundant variant in the human genome and are the most common source of genetic variation, with more than 10 million SNPs present in the human genome. They can also serve as biomarkers. |
| Somatic | Somatic cells include stem cells, blood cells, and other cell types. Changes to somatic cells are not permanent, meaning they cannot be passed down by generation. Somatic cell mutations include acquired alterations that can result from chemical or radiation exposure. Changes may also occur as cells are copied during growth or repair processes. |
Benefits of Genomic Medicine
Genomics in Healthcare and Medicine Today
Despite being a relatively new field, genomic medicine has impacted diagnostics and treatment in a significant manner.
It’s also been serving as a decision-making tool for many healthcare professionals.
But it’s important to know that despite the abundance of information our genome can provide us, our ability to understand it is still developing.
The more and more we learn about it, the more impact it will have on healthcare.
Genome sequencing is currently employed in a few healthcare fields, like cancer stratification, precision medicine, diagnosis and characterization of genetic diseases, and drug development.
Genomic Medicine in Cancer
In recent years, scientists worldwide have been trying to identify genetic changes associated with several types of cancer to determine their role in tumor development and metastasis.
There has also been an ongoing attempt to use these findings to fight cancer.
Genomic medicine can help understand three important aspects of cancer.
- Molecular basis of cancer growth - what proteins are activated or silenced in cancer cells that have contributed to their uncontrolled growth.
- The metastatic ability - the spread of cancer cells from the place where they first formed to another part of the body.
- Drug resistance - not all drugs will be effective against a type of cancer, and not all patients with the same cancer will respond in the same way to the same treatment.
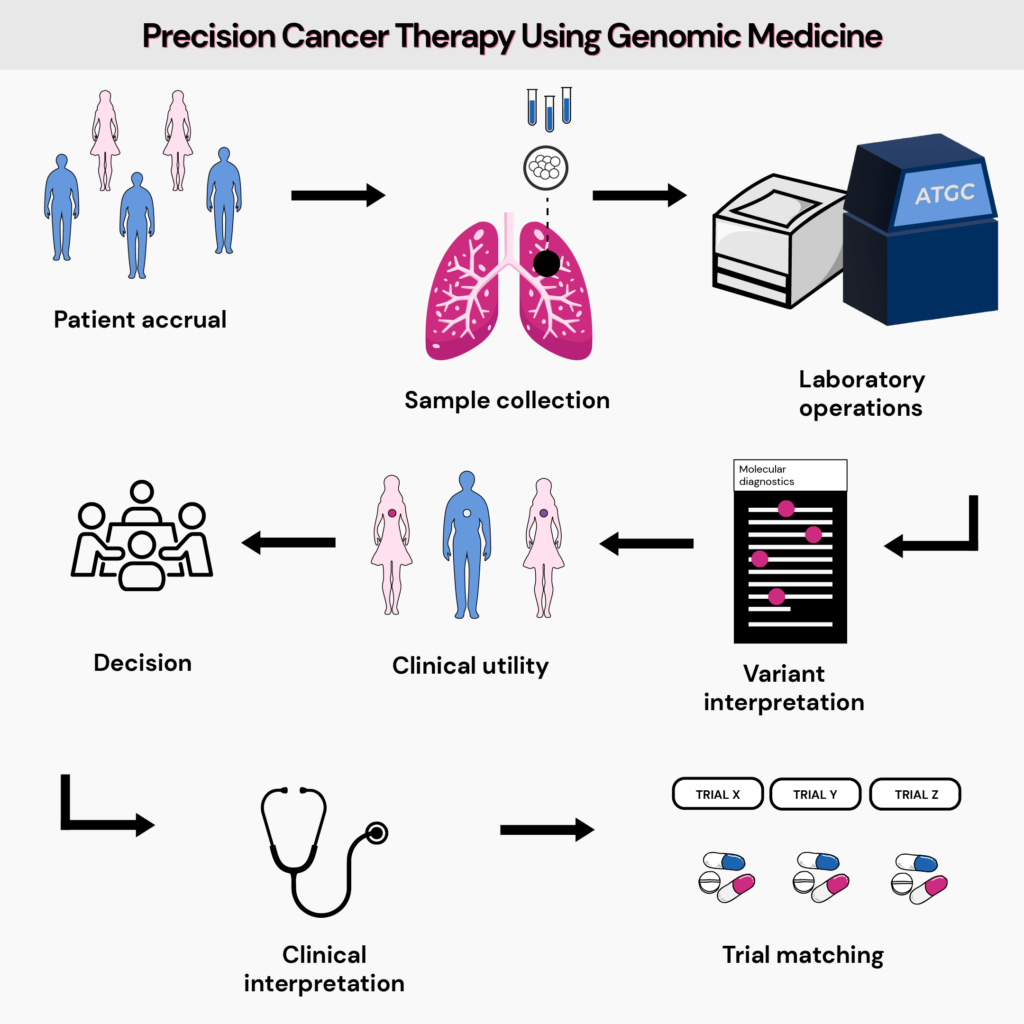
Precision medicine is cancer, most often means looking at how changes in certain genes in a person's cancer cells might affect their care, such as diagnosis, treatment, and other management options.
It has allowed clinicians to classify tumors based on mutations and responses to drug therapies.
This allows drug development that can fight cancer in more than one way.
- Inhibit enzymes that contribute to abnormal cell growth.
- Block the functioning of certain genes in cancer cells.
- Stop molecular pathways that help cancer cells thrive.
Such targeted therapies also help overcome the severe side effects of chemotherapy to an extent.
Since the treatment can be designed to target certain characteristics present only in cancer cells and not normal cells, they are less toxic to the patients.
The following are some examples of drugs developed using precision medicine:
- Imatinib (Gleevec) - for leukemia
- Trastuzumab (Herceptin) - for breast cancer
- Erlotinib (Tarceva) and gefitinib (Iressa) - for lung cancer
Genomic Medicine in Drug Prescription and Development
Using genomic information to analyze how a person’s genes affect their response to medication is called pharmacogenomics.
Before the advent of pharmacogenomics, drug development followed the “one-size-fits-all” approach.
Genomic medicine has now challenged this idea by bringing into light the different ways a person’s genetic makeup can affect drug responses.
Pharmacogenomics allows your doctor to identify the drugs that’ll likely work for you and the optimal dosage.
This applies to various classes of drugs, including antidepressants, opioid pain relievers, heart medications, anti-inflammatories, anti-diabetics, and medications used before and after surgery.
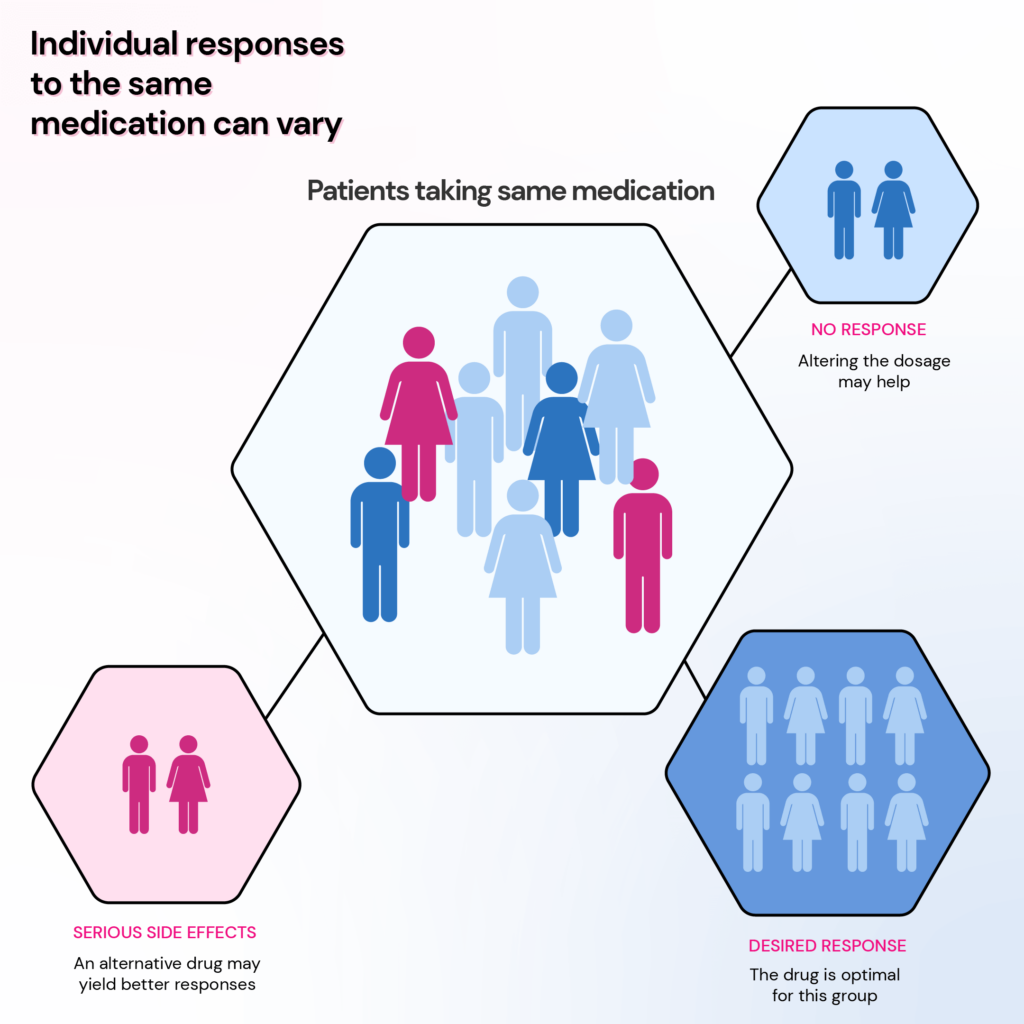
Current Use Of Pharmacogenomics
Breast Cancer and Trastuzumab - HER2
In some types of breast cancer, there are too many HER2 receptors.
Trastuzumab can treat only these types since it works by attaching to the HER2 receptors on cancer cells and killing them.
HIV and Abacavir - HLA-B
Abacavir is an effective treatment for AIDS since it fights against HIV.
Research suggests that about 5-8% of the people undergoing this treatment experience a hypersensitivity reaction, which manifests as rash, fatigue, and diarrhea.
This is due to an exaggerated response to the drug by the immune system.
Studies that explored the association between genes that regulate the immune system and abacavir hypersensitivity have discovered that a type of HLA gene called HLA-B*5701 increases the risk of hypersensitive reactions.
Those carrying this gene may benefit better from alternative drugs.
Cholesterol and Statins - SLCO1B1
Statins are a class of drugs that lower blood cholesterol levels.
Certain transporter proteins made by the SLCO1B1 gene carry statins to the liver, where they function to remove excess cholesterol.
People with a certain genetic change in the SLCO1B1 (*5) may experience muscle problems like weakness and pain since this change results in lower levels of simvastatin taken to the liver.
Higher levels of statin in the muscles can cause statin-induced myopathy
Observational and patient registry studies report a 7% to 29% incidence of statin-associated muscle symptoms (SAMS).
The risk of SAMS in people carrying the *5 type of SCLO1B1 gene is the highest with simvastatin and the least with pravastatin or rosuvastatin.
Thus, pharmacogenetic testing for this gene allows tailoring statin therapy based on genetics.
Blood Clots and Warfarin - VKORC1
Warfarin is a blood-thinning drug (anticoagulant) used to prevent heart attacks and strokes.
It works by interfering with the activity of an enzyme involved in blood clotting called the vitamin K epoxide reductase.
A gene called VKORC1 strongly influences warfarin dosing.
It produces vitamin K epoxide reductase, which is the target for warfarin.
People with a certain type of the VKORC1 gene have an increased sensitivity to warfarin and require a lower starting dose.
Certain enzymes in the CYP group, like CYP2C9, CYP3A4, and CYP1A2, play a role in warfarin pharmacogenomics.
Genomic Medicine In Predicting Disease Risk
Scientists are discovering that millions of people are living with an increased risk for certain serious health conditions without signs or symptoms due to small changes in their DNA.
Thanks to genomic medicine, it is possible not just to identify these variants but also to predict their effect to prevent these conditions years before symptoms appear.
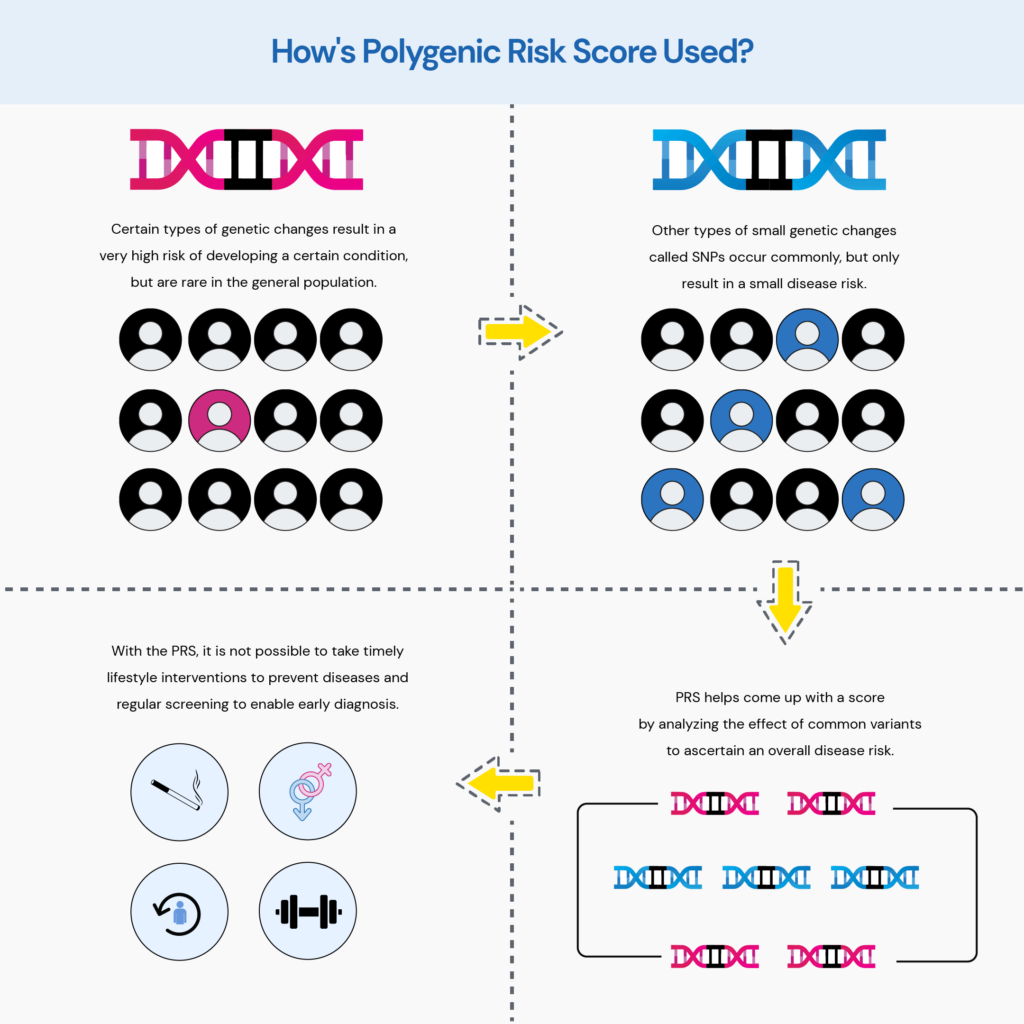
Many common conditions are typically not caused by just a single mutation.
The risk is due to millions of inherited variants called SNPs, each of which contributes only a little to the disease risk.
But when we add the effect of these small changes, it can greatly impact an individual’s health profile.
Polygenic risk scores (PRS) are promising tools for predicting disease risk.
PRS calculates the sum of the effects of different variants to come up with a score that indicates a person’s risk for a particular health condition.
This information can allow physicians to devise effective preventive strategies and closely monitor high-risk individuals for early diagnosis.
Gene Health Report: Identify your risk for 47 chronic diseases for just $50
What Does The Future Have for Genomic Medicine In 2024?
It is safe to say that genomics is changing how doctors practice medicine and treat diseases.
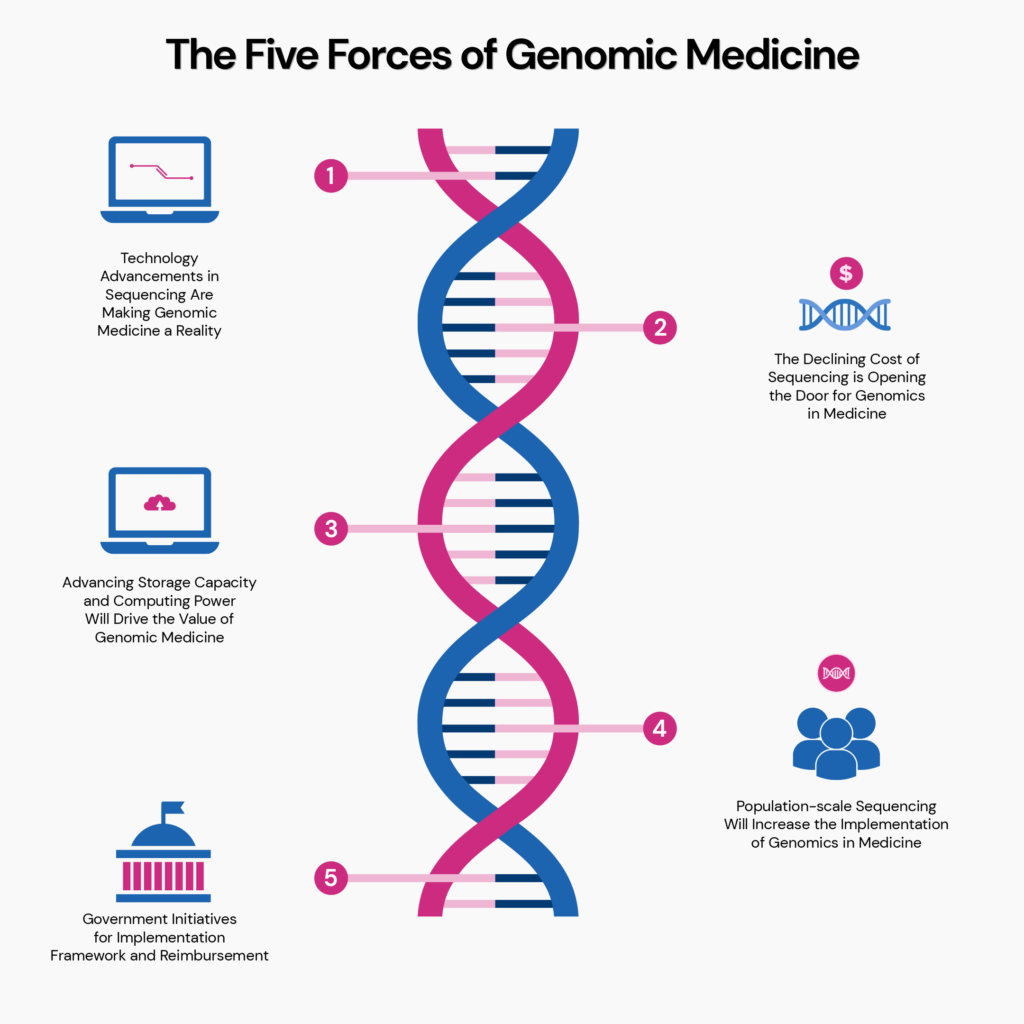
According to a new white paper by BIS Research titled The Five Forces of Genomic Medicine, “genomic medicine has the potential to save lives, transform medical practice around the world, and drive billions of dollars of economic activity.”
The National Human Genome Research Institute (NHGRI) developed strategic engagement to identify future research priorities and opportunities in human genomics, emphasizing health applications in 2020.
Here is a summary of 10 bold and fantastical predictions made by experts at the NHGRI for the next decade.
| 10 Predictions About The Future Of Genomics By The NHGRI |
| Whole genome sequencing and analysis will become a normal routine in any research lab. |
| The function of every gene in human DNA will be discovered and understood. |
| The effects of the environment on gene function will be routinely taken into account to predict disease risk and health outcomes. |
| Studies will abandon race and other social constructs as biological categories in genomic research. |
| Students will regularly display projects regarding genome sequence studies as a part of their school science fairs. |
| Genetic testing will soon become a commonly used medical tool like blood tests. |
| Geneticists will readily be able to tell whether a variant is clinically relevant. |
| Each individual’s whole genome sequence will be accessible via smartphones in a user-friendly format. |
| Genomic advances will benefit not just certain people or communities but society as a whole. |
| Discoveries and new technologies in genomics will help cure many more genetic diseases. |
What We May See In 2024
Pharmacogenomics in Psychiatry: Genetic testing can help identify individuals who may have a poor response or heightened risk of adverse reactions to certain psychiatric medications, such as antidepressants and antipsychotics. This information can guide clinicians in selecting the most effective medication and dose for each patient, improving treatment outcomes, and minimizing side effects.
Digital Health Technologies: Integrating genomic information with digital health technologies opens new avenues for personalized healthcare. Genomic data can be combined with wearable devices, smartphone apps, and telemedicine platforms to provide real-time monitoring, proactive health management, and personalized interventions. For example, genetic information can be used to create personalized exercise and nutrition plans tailored to an individual's genetic attributes and health goals.
Microbiome-based Therapies: The human microbiome, comprising trillions of microbes living in and on our bodies, significantly influences our health and disease susceptibility. Researchers are exploring the potential of microbiome-based therapies to treat various conditions, including gastrointestinal disorders, metabolic disorders, and even mental health disorders. By understanding the interplay between an individual's genetic makeup and their unique microbiome composition, personalized microbiome interventions can be developed to restore a healthy microbial balance and promote overall well-being.
Economic Benefits of Genomic Medicine
The average lifespan of the human population is increasing.
According to the World Health Organization, life expectancy has increased globally by more than 6 years between 2000 and 2019 – from 66.8 years in 2000 to 73.4 years in 2019.
In this landscape, genomic medicine will transform healthcare to increase this number further.
Having genomic information at hand means having information about your health and well-being and the ability to make informed choices.
On the personal economy side, the resulting longer health span can increase a person’s earning capacity.
Further, with improved disease risk identification tools, individuals reduce their health costs through early detection and avoiding unnecessary treatments.
Genomic information can also help impact the national economy by cutting productivity loss and disease treatment costs.
Should You Have Your Genome Sequenced?
With the continually declining costs of whole genome sequencing and swift results, the number of people who opt to get this done is on the up and above.
While opting for genome sequencing is a very personal choice, it could be worthwhile to be aware of its concerns.
- Though the sequencing cost may be around $300-$1000, there could be significant additional costs for interpretation and counseling. Not many health insurance cover these costs.
- The whole preventive model of genomic medicine is based on behavioral and lifestyle changes to mitigate disease risk. So it would serve well to ask yourself, "will I be able to follow recommendations and adhere to lifestyle changes in the long run?"
- There's also a chance that while you're getting your genome sequenced to look for a specific health condition, you may encounter an incidental finding about an unrelated condition with potentially alarming consequences. For instance, you may be interested in having a genetic risk assessment for heart diseases. But your results may end up additionally revealing a high risk for Alzheimer's. However, some research institutes and companies have policies regarding such incidental findings, and having this information can help prepare for the test better.
- Since you share your DNA with your family members, your findings may apply to some family members. Therefore, it can be a difficult moral dilemma to make a decision about disclosing this information to concerned people.
- A commonly expressed concern regarding direct-to-consumer (DTC) genetic testing is the privacy of DNA data. Many reports reveal that several DTC genetic companies sell customers' data to pharmaceutical companies. It is vital for companies to lay down strict privacy policies and for users to have a detailed look at them before getting a test.
- Lastly, we still have a long way to go in understanding all the information our genome can provide us. While your whole genome sequencing files may occupy several gigabytes of your PC memory, a very small percentage may be relevant to your health.
The bottom line, genomic medicine can transform medicine and healthcare.
The medical and scientific communities worldwide are just beginning to seize the transformative opportunities.
We have just scratched the surface of genomic medicine, and a mountain of information is waiting to be discovered.
References
- https://www.genome.gov/about-genomics/fact-sheets/Genetics-vs-Genomics
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6579593/
- https://www.genome.gov/health/Genomics-and-Medicine
- https://www.cancer.gov/about-nci/organization/ccg/cancer-genomics-overview
- https://medlineplus.gov/genetics/understanding/genomicresearch/pharmacogenomics/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3000684/
- https://www.ncbi.nlm.nih.gov/books/NBK315783/
- https://www.healthline.com/health/what-is-statin-induced-myopathy-or-muscle-pain
- https://www.ahajournals.org/doi/full/10.1161/CIRCGEN.118.002320#R11
- https://www.ncbi.nlm.nih.gov/books/NBK84174/
- https://www.genome.gov/Health/Genomics-and-Medicine/Polygenic-risk-scores
- https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6136295/
- https://pubmed.ncbi.nlm.nih.gov/29801579/
- https://www.sciencedirect.com/science/article/pii/S0002929722002075
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6568267/